In general life, the classification of things as per their size, shape, usage and other sorting criteria is important to live systematically. Similarly, the classification of elements has always been considered important in order to understand similarities, reactabilities and compounding possibilities of the elements. The 19th century and early 20th century witnessed considerable progress in the field of classification of elements.
Dobereiner’s Law of Triads
In 1817, German scientist J W Dobereiner proposed that the elements occur in groups of three when arranged in increasing order of atomic mass. He called every such group a triad. According to him, the atomic weight of the middle element is almost same as the average of the atomic weights of the other two elements. According to him, all the elements of a triad have similar chemical properties.
Example: Triad 1
| Element | Lithium (Li) | Sodium (Na) | Potassium (K) |
| Atomic Mass | 7 | 23 | 39 |
The average of masses of Lithium and Potassium gives the atomic mass of Sodium. All the three elements have similar chemical properties.
Newland’s Law of Octaves
In 1864, English chemist John Newlands presented tabular classification according to increasing order of their atomic masses. He arranged the elements in groups of seven as according to him, every eighth element in the table showed physical and chemical properties similar to that of the first one. His arrangement of elements was called octave.
Example:
| Li | Be | B | C | N | O | F |
| Na | Mg | Al | Si | P | S | Cl |
| K | Ca |
The physical and chemical properties of Li, Na and K are similar to each other.
Mendeleev’s Periodic Table
In 1869, Russian scientist Dmitri Mendeleev proposed the most successful classification of elements in order of increasing atomic weights. According to him, “properties of elements are periodic function of their atomic weight.”
Mendeleev explained that the gaps in his table are for yet to be discovered elements. His claim proved right with discovery of Gallium which he had kept as undiscovered eka-aluminium and scandium which he kept as eka-boron.
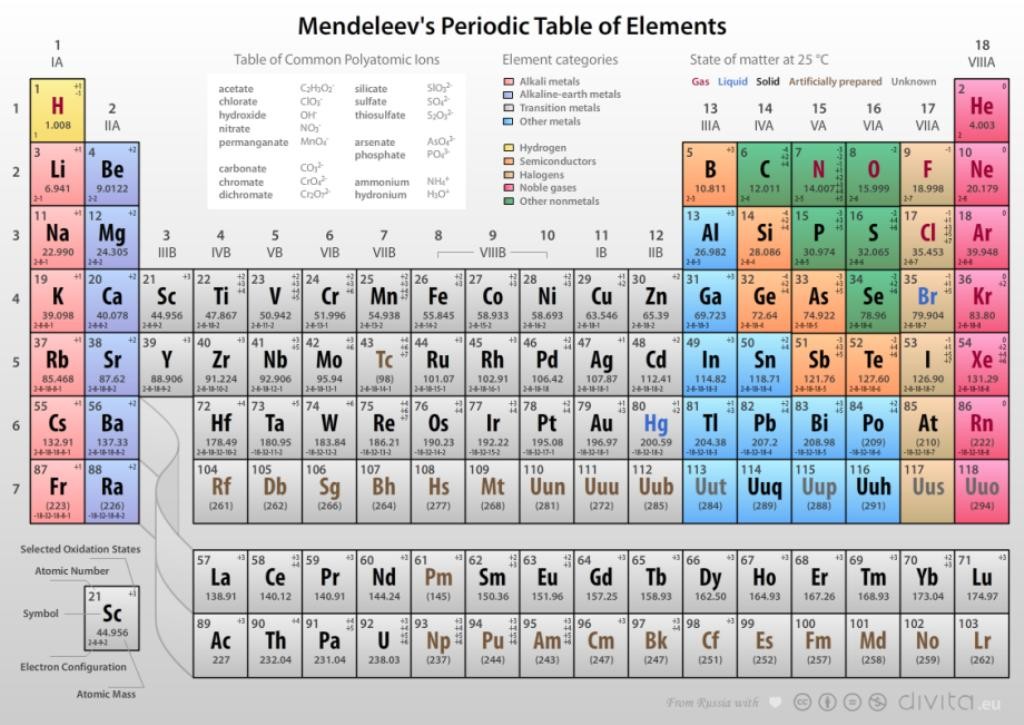
Mendeleev Periodic Table
In Mendeleev’s table there are 6 horizontal rows known as periods and 8 vertical rows called as groups. Elements in one group have similar properties.
Henry Moseley’s Modern Periodic Table
In 1913, British scientist Henry Moseley proposed that the elements should be arranged in order of increasing atomic numbers instead of atomic weights. According to him, properties of elements are a periodic function of their atomic numbers.
According to the modern periodic law, if the elements are arranged in order of their atomic numbers then the elements with similar properties are repeated at a regular interval.
The modern periodic table has 7 horizontal rows or periods and 18 vertical columns.
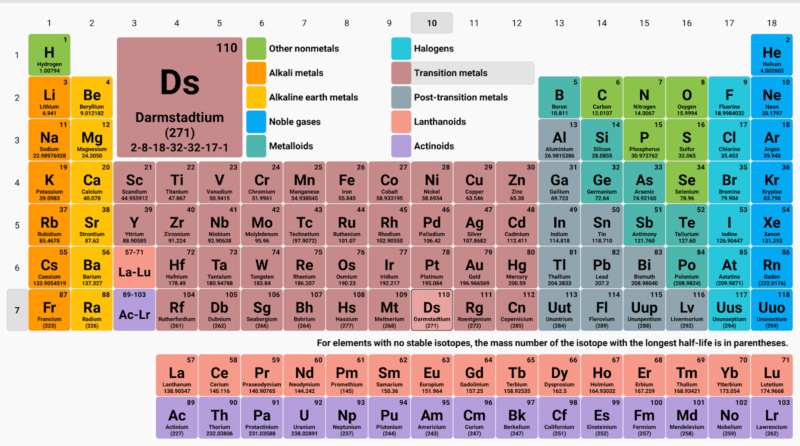
Modern Periodic Table
Trends in modern Periodic Table
-
Valency and electronic configuration
All elements in a group have same valency. The group number represents the valancy with respect to oxygen.
Moving across a period from left to right the valency increases from 1 to 8.
-
Atomic Size
Atomic size increases while moving downwards in a group. While moving from left to right in a period, the atomic size decreases.
-
Metallic and Non-metallic Properties
While moving downwards in a group the metallic property increases gradually. While moving from left to right in a period, metallic properties gradually decrease. It means that the elements on the left are true metals and on the right are true non-metals.
-
Electro-negativity
While moving downwards in the group the tendency of gaining an electron decreases.
NCERT Solutions for Class 10 Science chapter 5 – Periodic Classification of Elements











