State Board Commerce (XI-XII) - Test Papers
Albert Einstein
Albert Einstein, a theoretical physicist from Germany stands as the strong foundation to the modern physics. His theory of relativity is the pillar of the physics. He is also known as a great philosopher.
Einstein is revered universally for his theories and equations. The three major works include,
- Einstein Field Equation
- Theory of the relativity– the world’s most popular equation and
- Law of photoelectric effect
His theories and discoveries laid the foundation for developing quantum theory.
Work Profile
After graduation, who excelled in Math and science, struggled a couple of years to get a teaching job. He joined as a Patent Clerk in Swiss Patent Office, in Bern to have a steady flow of income. Working as a clerk, he embarked a great journey and explored an extraordinary career in the world of science.
He earned his doctoral degree in the year 1905. He published four scientific papers, which has its significance even after 100 years. He introduced the new and special concepts of Photon theory of light and special relativity.
Explanation of the Einstein photoelectric effect earned all the essential attention towards him. It literally shook the whole world.
Einstein Field Equation
Einstein field of equation, precisely termed as EFE, it is a set of 10 equations derived by Albert Einstein. Einstein field equation explained here.
The equation denotes the general theory of relativity founded by Albert Einstein, which describes the basic interaction of gravity towards spacetime, when curved by energy and mass.
The EFE is written as
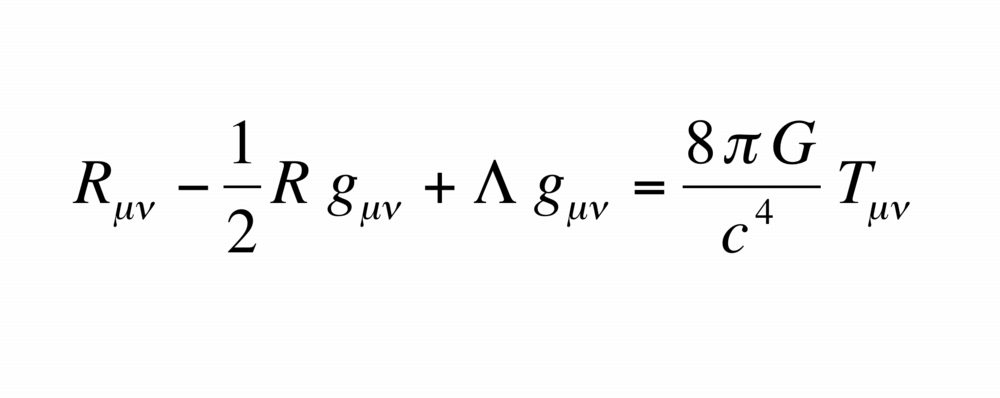
The tensor in EFE relates to a set of 4 x 4 symmetric tensors. Each tensor composes of 10 independent objects.
Explanation of photoelectric effect
The photo electric effect is a common phenomenon, where the metal surface emits the electrons upon light of adequate frequency. It was founded by Heinrich Hertz in the year 1887. Later, in 1092, Lenard also found it. However, electromagnetic theory of Maxwell could not connect with the both their observations of the photoelectric effect.
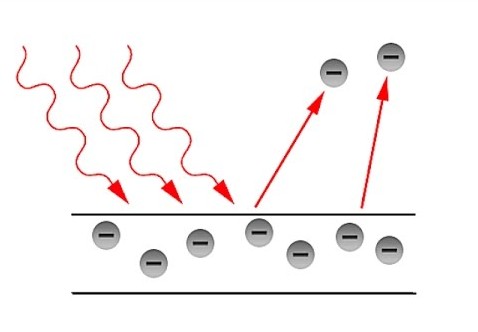
The view of Einstein photoelectric effect has an easy explanation and solved this problem.
He used Planck’s idea. According to the revolutionary idea, light is considered a particle. The energy level carried by any particle is based on the frequency of the light. Here, light is a particle and carries photon or quanta.
He framed an equation for the Einstein photoelectric effect E = hv
- E represents Energy
- h is Planck’s constant
- v is frequency of the energy radiated
Theory of Relativity
E = mc2!
The equation, E = mc2 depicts that mass and energy are interchangeable. It was developed by Einstein in the year 1905. This theory of relativity forms the foundation of the modern physics.
Probably a confusing theory of Einstein that most students may fail to understand the core. Theory of relativity simplified here.
The theory determines that the laws of physics apply equally to all non-accelerating observers. He demonstrated with an example:
Speed of the light in a vacuum remains the same regardless of the speed in which the observer travel.
NCERT Solutions for Class 10 Science Chapter 5 – Periodic Classification of Elements
In general life, the classification of things as per their size, shape, usage and other sorting criteria is important to live systematically. Similarly, the classification of elements has always been considered important in order to understand similarities, reactabilities and compounding possibilities of the elements. The 19th century and early 20th century witnessed considerable progress in the field of classification of elements.
Dobereiner’s Law of Triads
In 1817, German scientist J W Dobereiner proposed that the elements occur in groups of three when arranged in increasing order of atomic mass. He called every such group a triad. According to him, the atomic weight of the middle element is almost same as the average of the atomic weights of the other two elements. According to him, all the elements of a triad have similar chemical properties.
Example: Triad 1
| Element | Lithium (Li) | Sodium (Na) | Potassium (K) |
| Atomic Mass | 7 | 23 | 39 |
The average of masses of Lithium and Potassium gives the atomic mass of Sodium. All the three elements have similar chemical properties.
Newland’s Law of Octaves
In 1864, English chemist John Newlands presented tabular classification according to increasing order of their atomic masses. He arranged the elements in groups of seven as according to him, every eighth element in the table showed physical and chemical properties similar to that of the first one. His arrangement of elements was called octave.
Example:
| Li | Be | B | C | N | O | F |
| Na | Mg | Al | Si | P | S | Cl |
| K | Ca |
The physical and chemical properties of Li, Na and K are similar to each other.
Mendeleev’s Periodic Table
In 1869, Russian scientist Dmitri Mendeleev proposed the most successful classification of elements in order of increasing atomic weights. According to him, “properties of elements are periodic function of their atomic weight.”
Mendeleev explained that the gaps in his table are for yet to be discovered elements. His claim proved right with discovery of Gallium which he had kept as undiscovered eka-aluminium and scandium which he kept as eka-boron.
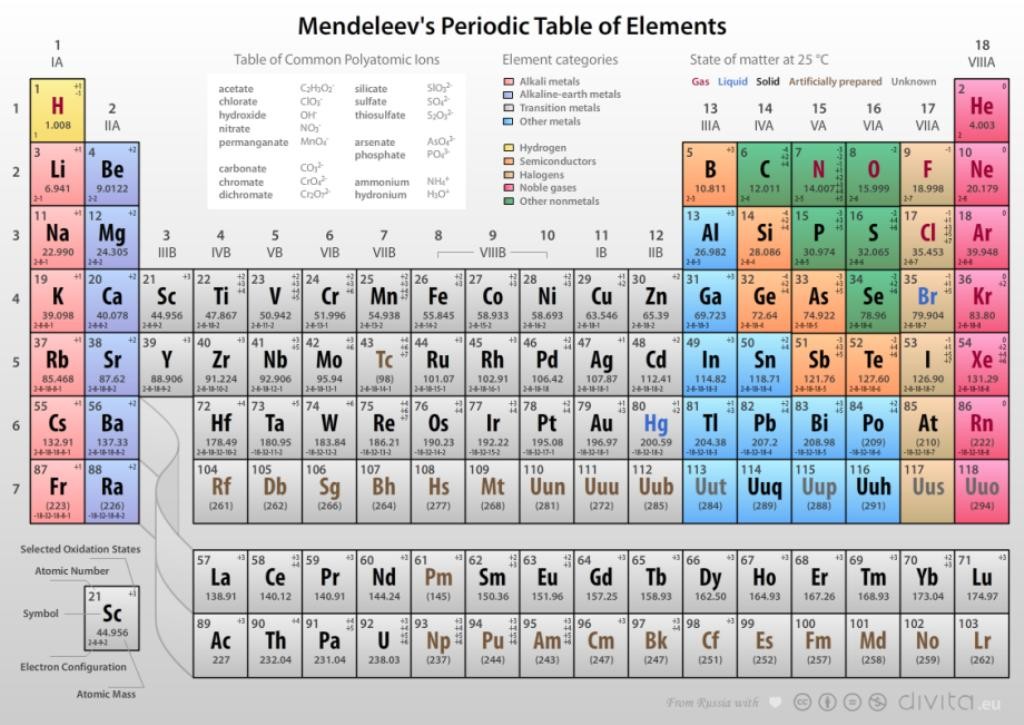
Mendeleev Periodic Table
In Mendeleev’s table there are 6 horizontal rows known as periods and 8 vertical rows called as groups. Elements in one group have similar properties.
Henry Moseley’s Modern Periodic Table
In 1913, British scientist Henry Moseley proposed that the elements should be arranged in order of increasing atomic numbers instead of atomic weights. According to him, properties of elements are a periodic function of their atomic numbers.
According to the modern periodic law, if the elements are arranged in order of their atomic numbers then the elements with similar properties are repeated at a regular interval.
The modern periodic table has 7 horizontal rows or periods and 18 vertical columns.
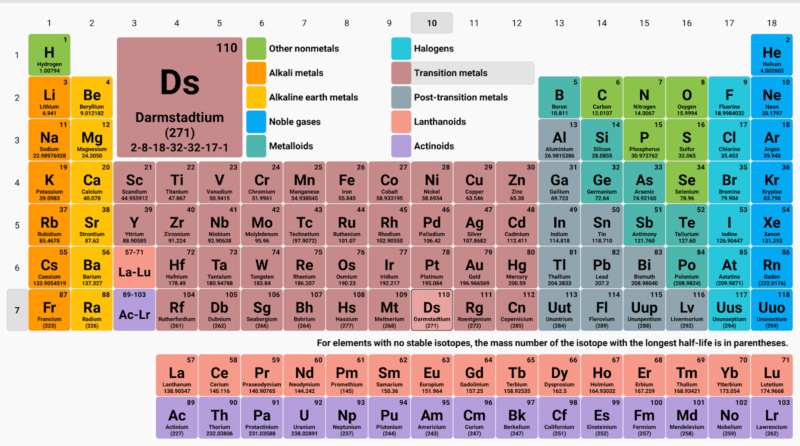
Modern Periodic Table
Trends in modern Periodic Table
-
Valency and electronic configuration
All elements in a group have same valency. The group number represents the valancy with respect to oxygen.
Moving across a period from left to right the valency increases from 1 to 8.
-
Atomic Size
Atomic size increases while moving downwards in a group. While moving from left to right in a period, the atomic size decreases.
-
Metallic and Non-metallic Properties
While moving downwards in a group the metallic property increases gradually. While moving from left to right in a period, metallic properties gradually decrease. It means that the elements on the left are true metals and on the right are true non-metals.
-
Electro-negativity
While moving downwards in the group the tendency of gaining an electron decreases.
NCERT Solutions for Class 10 Science chapter 5 – Periodic Classification of Elements
NCERT Solutions for Class 10 Science Chapter 2 – Acids, Bases and Salts
NCERT solutions for class 10 Science chapter 2 cover the important topic of acids, bases and salts. The acids and bases are important elements in any chemical reaction. These chemicals are used in our day to day life. Some of the commonly used acids are vinegar (ethanoic acid) and lemon juice (citric acid) and salt is common salt.
The acids and bases can either be concentrated or dilute. Here we brief about the same
Acids
They turn blue litmus paper to red. Acids contain hydrogen ions and react to form salts with metals. They have a pH less than 7. The acids found in lab are Hydrochloric acid, Sulphuric acid or Nitric acid
Bases
They turn red litmus paper to blue. Alkalis contain hydroxide ions (OH-). The bases found in lab are Sodium hydroxide, Calcium hydroxide or Ammonia.
Properties of Acids and Bases
Properties of acids
- All acids are liquid in nature
- They are solutions in water
- They are sour to taste
- Corrosive in nature
- Turn Universal Indicator from green to red
Properties of alkalis
- They produce soap or lather
- Soluble bases
- They can burn skin
- Bitter to taste
- Turns Universal Indicator from green to blue or purple
Salts
They are formed by interaction of acid with metals. The H+ ion from acid gets replaced by the metal ion resulting in salt.
Common Salt (Sodium Chloride)
Sodium chloride (NaCl) is also known as common or table salt. It is formed by reaction between sodium hydroxide and hydrochloric acid. It is a neutral salt i.e. the pH value of sodium chloride is 7. It is used to add taste to food.
Chemicals from Common Salt
It can also be used in the manufacturing of various chemicals such as sodium hydroxide, Sodium carbonate, bleaching powder etc.
Download NCERT Solutions for Class 10 Science Chapter 2 – Acids, Bases and Salts
NCERT Solutions for Class 10 Science chapter 3 – Metals and Non – Metals
Mrs. Revathi Srinivasan talks about the Advantages of Technology in Education
Mrs Revathi Srinivasan, Director – Education and Principal, Smt. Sulochanadevi Singhania School answers questions regarding the growing use of technology in today learning process. Is it a boon or a bane? Behram Sir of Robomate+ asks questions and doubts on behalf of many educationists, teachers and parents.
8 Tips on How to Focus on Studies and Get Ready for Exams
Studying for hours on end is not necessarily productive. However, learning for a small duration, but with concentration is much better. The main aim of learning is to grasp concepts and information and remember it. If you try to learn without understanding, you will end-up trying to memorize chapters, which is really not easy, and ultimately forget what you have memorized soon. Here we give you tips on How to Focus on Studies.
How to Stay Focused While Studying
Organize and Make a Plan to Study
Make a proper study timetable and stick to it on daily basis. Make a list of chapters or concepts you need to revise and paste it on your wall, so you will know exactly the things that have to be done.
Set Daily Goals that are Achievable
Sticking to a timetable can be a very daunting task. So make a goal oriented time table rather than just a list of things to be done. Try to set specific study goals which are achievable like the number of pages you will learn or the number of math problems you will solve. Make sure you complete your tasks for the day before going to bed.
Read: 5 Tips To Get Most Out Of Your Study Time
Stay Away from Distractions
According to a study done by University of California-Irvine, it can take, on average, nearly 20 minutes to get back to the earlier level of concentration after an interruption. Electronic gadgets are the main source of distraction today. Social Media including video sites kill precious time of students. Studying in a place where the television is being watched or other children are having fun will lead to unwanted distractions. So staying away from all distractions is a good way to stay focused while studying.
Read: Tips to Balance Your Study and Play Time
Study in a Proper Study Environment
Fix a place for your study. Maybe it is your room, outhouse or a room on your terrace. You can paste charts of formulas, diagrams, time table, etc, to refer and memorize them easily. The place should be away from all kinds of distractions including your television and radio. It should give you peaceful environment to study. A desk or table facing your charts and flash cards will lend the room an environment that encourages longer periods of focus while studying. Avoid studying on your bed since you may get the urge to lie down and then doze off.
Note Important Tasks if They Come to Mind
Many times while you are doing something important, in this case – studying, you remember an important task that you had to do, but forgot. Don’t rush to do it right away. It will take away precious minutes of your allotted time of study. Instead, make a list of to do list which you will do after you have finished your studying.
Sufficient Rest and Sustenance is Part of the Study Process
Sufficient sleep, healthy food and exercise is necessary to have a healthy mind and body that supports your intentions of studying according to plan and completing goals you’ve set in your time table. An empty stomach can also be a distraction. A restless brain and a weak body will not help in staying focused while studying.
Keep all required things with you (water, Snacks etc.)
Along with the best study material and study tools you can keep lots of fresh fruits and vegetables snacks which help in increasing memory power and stamina. You should keep yourself hydrated with water or fresh fruit juices. Always have a bottle of water on your study table to avoid getting up to fetch water.
Difficult to Concentrate for Long Periods
The biggest problem to concentrating on anything is lack of interest. Studies for many is not something they want to do. So you will have to motivate yourself to study. Remember good marks will get you admiration from classmates and adulation from friends and relatives. Also you could begin your time-table setting with smaller periods of time between breaks. You could sit continuously for 30 minutes at the start and build up to a couple of hours over a period of days.
Best Reference Book for SSC Board Exam Class 10
Class X is the major milestone for students and they are required to study hard to score well. There are a number of books available in the market to refer and write Maharashtra Board exams such as Navneet, target, Unique Solutions, or Master key
Introduction:
Master key is the most popular book series among students. The books are published under Chetana Publications. It publishes study material for Class V to X for students of both English & Marathi medium. It covers all the subjects of English & Marathi mediums. In addition, to the high school books, it also has a book series of XI & XII Science & Commerce streams.
Why Master Key?
Master Key is the best book for class X Maharashtra board, here we give you points which differentiate and supersede it from other book series
- Summary of Every Chapter: Each chapter starts with the summary providing insights about the topics which will be covered. This helps students to be prepared for the sub-topics to be studied
- Easy Understanding: The topics are explained in simple language in a stepwise manner. The students find it easy to understand hence can concentrate and learn better.
- Answers in Points Form: Long paragraphs are boring and difficult to understand. Splitting the answers in a pointwise manner helps in easy learning. The book provides diagrams and charts wherever applicable
- Similar to Textbook: Master Key covers all the topics as per the Maharashtra Board class X, making it the best book for class X Maharashtra board. All the topics are efficiently covered to get score in the board exams.
- Practice Paper After every Chapter: All the important questions which has appeared in the previous year question Paper are covered after every chapter.











